
Accelerating Digital Health Innovation for Big Pharma
Vynyl partnered with Novo Nordisk's Digital Transformation & Innovation team to systematize the discovery, prioritization and validation of new opportunities in digital health.
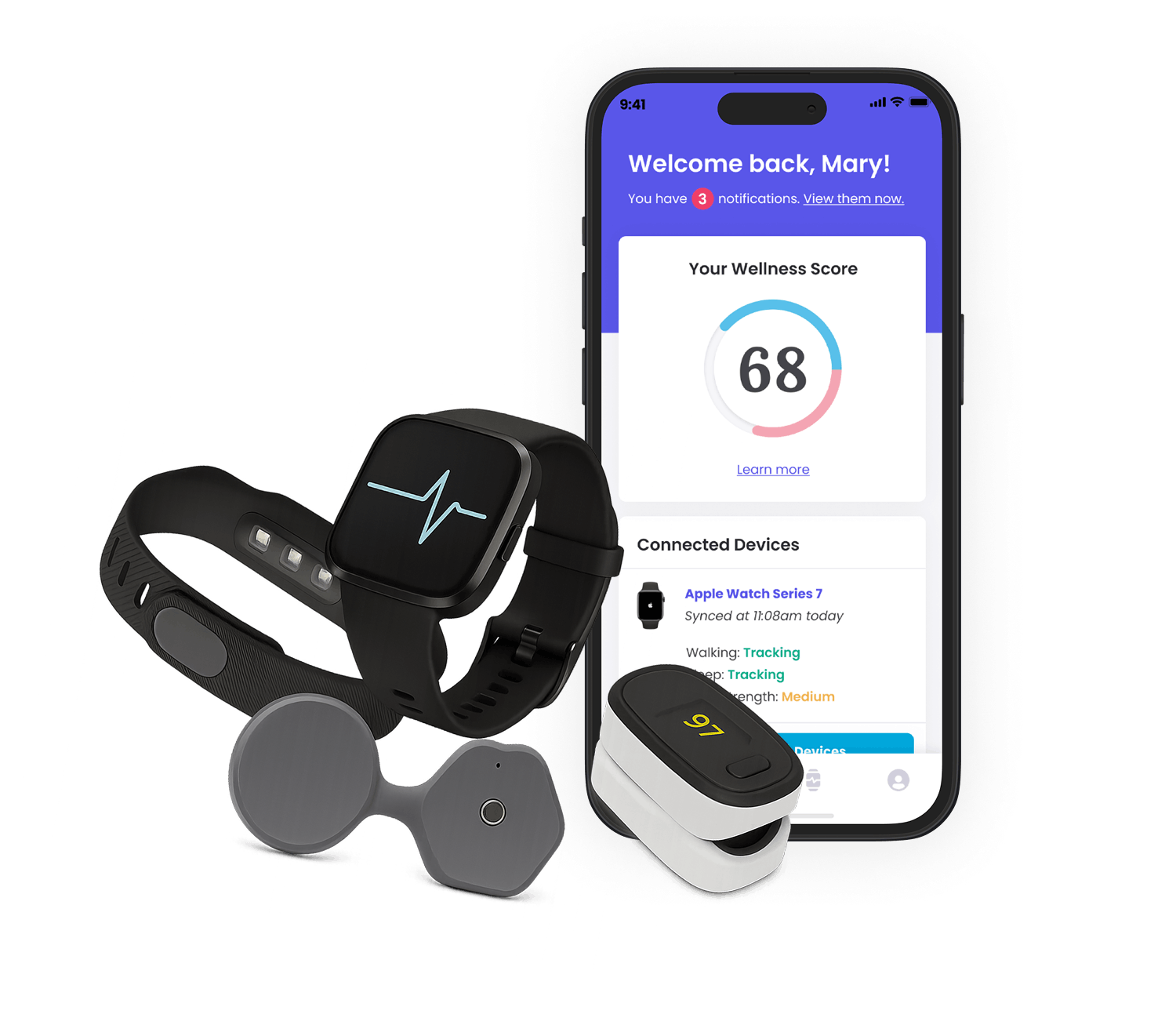
The Challenge
Novo Nordisk faced the classic innovator's dilemma: how to build digital capabilities that could match the excellence of their core business. Our challenge was to establish an innovation team that could not only generate compelling digital health concepts but also validate their viability with precision and speed.
The real challenge wasn't a lack of ideas—it was establishing a structured approach to prioritize opportunities, validate concepts rapidly, and secure crucial stakeholder buy-in across the organization. Without this foundation, promising innovations repeatedly lost momentum, languishing between departments or failing to demonstrate clear strategic value. What Novo Nordisk needed wasn't innovation theater, but a shift in how strategically valuable innovation was operationalized.
Our Approach
Working closely with Novo Nordisk's team, we helped refine an innovation framework that balanced rapid experimentation with pharmaceutical industry constraints. At the foundation of our approach was a deep understanding of shifting market dynamics and emerging technology paradigms. We brought precision and resourcefulness to research efforts, conducting thorough interviews with patients and experts, while maintaining alignment with compliance and legal, and always tracking our outcomes against our initial hypotheses and success criteria. From there, our passion for storytelling and executive communication allowed us to drive stakeholder buy-in when presenting findings to leadership teams.
This collaborative infrastructure supported over 50 in-depth clinical user interviews and 20+ quantitative validation studies, creating a rich ecosystem of insights that informed every stage of innovation.
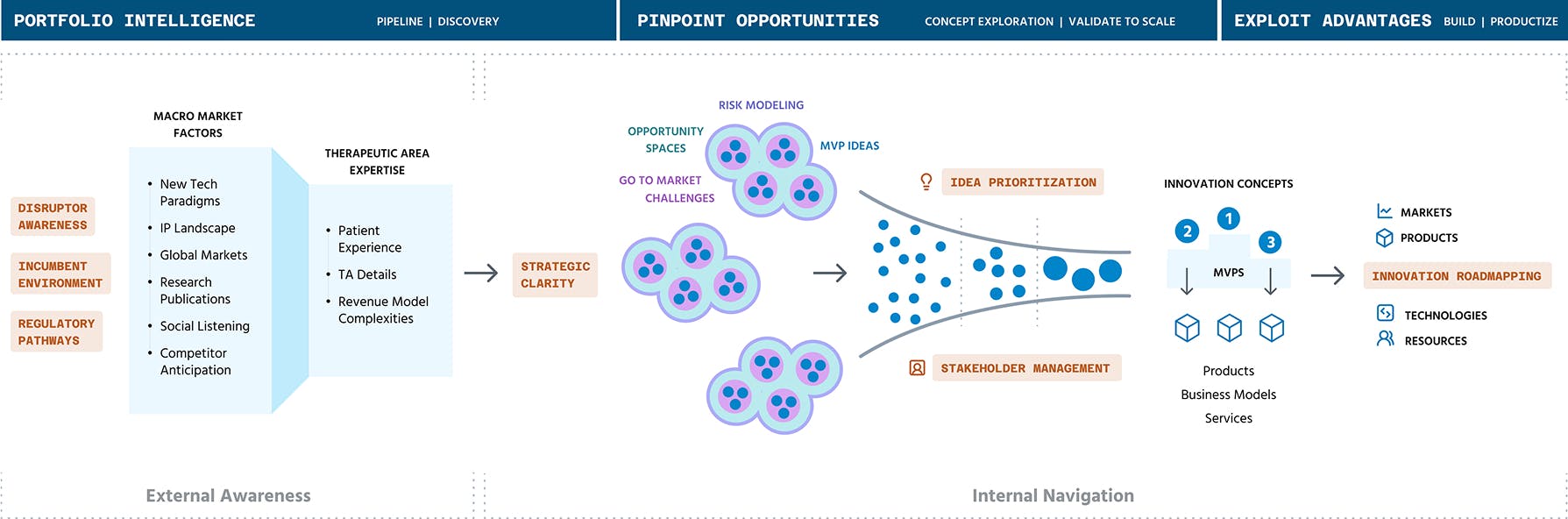
Key Takeaways
Our early recognition of AI's transformative potential in healthcare positioned us as thought leaders within Novo Nordisk. By anticipating these trends before they became mainstream, we helped the team develop a forward-looking innovation roadmap that prepared them for the coming wave of AI-driven healthcare solutions rather than reacting to it. This foresight established credibility with senior leadership and created opportunities to pursue cutting-edge initiatives.
Working effectively within healthcare's complex regulatory environment requires specialized expertise. We excelled at navigating compliance requirements while maintaining research momentum, creating structured protocols that satisfied legal requirements without sacrificing speed or insight quality. This balanced approach ensured patient data protection while enabling meaningful research that could quickly inform product decisions.
Rapid prototyping became our signature method for aligning diverse stakeholders around a unified vision. By quickly transforming abstract concepts into tangible demonstrations, we bridged the communication gap between technical teams, business leadership, and clinical experts. These prototypes served as powerful alignment tools, allowing stakeholders to experience potential solutions rather than just imagine them, dramatically accelerating buy-in and decision-making across organizational boundaries.
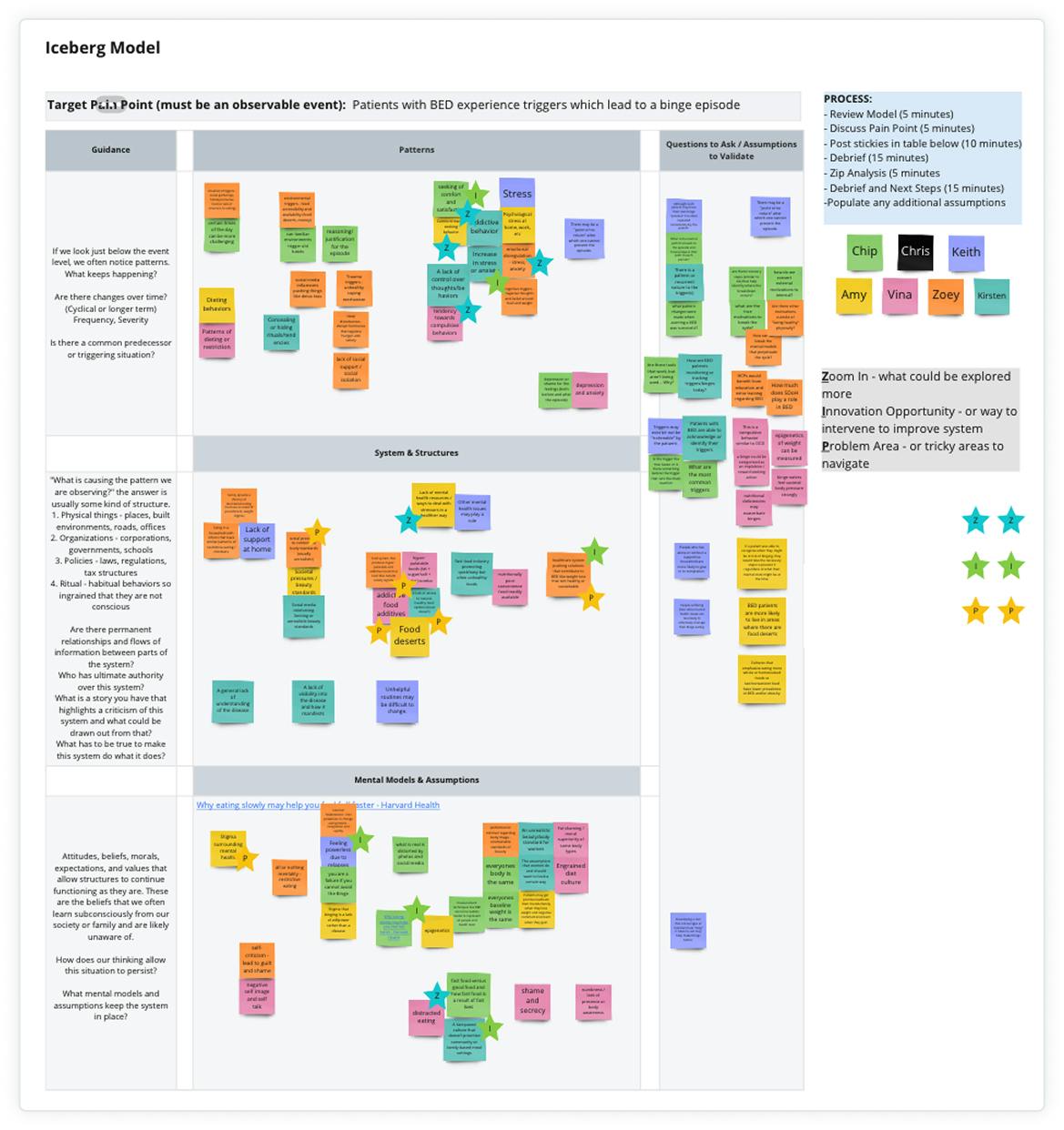
"Vynyl have been great partners because of their expertise. They have a lot of breadth of knowledge, but also a depth of knowledge. Not only within the innovation space, but also within healthcare and therapeutic areas."
The Result
The collaborative innovation approach delivered meaningful results across the organization. By continuously optimizing our processes, we helped galvanize enterprise-wide digital initiatives and established pathways for turning promising concepts into validated solutions. Multiple strategic innovation streams emerged across therapeutic areas, with numerous concepts advancing to well-crafted prototypes through efficient sprint methodologies.
The impact was substantial and measurable: validation cycles decreased dramatically, while successful pilot conversion rates showed real improvement. The streamlined processes enhanced regulatory confidence through consistent documentation, and the research infrastructure established across priority areas reduced feedback cycles from months to days.
Most importantly, the organization's innovation culture evolved significantly. We helped transform how the company approached digital opportunities—moving from isolated initiatives to a cohesive, strategic framework. Cross-functional collaboration became more effective, with commercial teams actively engaging in digital initiatives.
Looking Forward
Novo Nordisk now has a structured approach to identifying and validating digital opportunities, supported by robust research methodologies and validation processes that continue to evolve with the market.
The collaborative framework demonstrates how life sciences companies can effectively integrate digital innovation with their core business. As healthcare continues its digital transformation, Novo Nordisk is well-equipped to develop and validate new solutions that extend their impact beyond traditional medicine, while maintaining the rigorous standards essential in healthcare innovation. Their strengthened ability to align stakeholders, rapidly prototype concepts, and systematically validate opportunities has established a foundation for sustained digital innovation.
Take the Next Step
Let's transform vision into reality.
Schedule a conversation today and discover how partnering with Vynyl can turn your biggest challenges into market-defining advantages.



